Climate Change Global Warming
The Greenhouse Effect and Greenhouse Gases
In any discussion of the earth's climate, it is important to keep in mind the complexity of the system; there is a need to sacrifice detail in favor of some simplifying clarifications. Since the basic issue is heating of the earth and the concomitant rising temperature (0.74°C over the last century according to the 2007 Intergovernmental Panel on Climate Change or IPCC), it is appropriate to start with an understanding of the physics of temperature and heat. Temperature is a measure of the average kinetic energy of the particles in a sample of matter - how fast they are moving. When two objects of different temperatures are put next to each other, the faster movement of the hotter particles transfers to the slower, colder particles until they all have the same energy; they are moving at the same speed and therefore the same temperature, thermal equilibrium. The transfer of temperature from one object to another was determined to be a form of energy by J. P. Joule in the mid-nineteenth century; it has long been known as heat.
Heat is transferred from one place to another by one of three mechanisms: conduction; convection and radiation. Conduction is the heat of contact, what you feel when you put your hand on a hot or cold surface. Convection is the heat of flow, a colder fluid like air or water takes the heat away from a surface, like wind chill. Radiation is the heat of the sun, photons of varying energies transfer their energy to target molecules of a surface like earth's landmass, a fluid like earth's ocean or a gas like earth's atmosphere. The human body is subject to all three types of heat transfer: conduction of heat according to the ambient temperature of the air and ground at all times, convection of heat if there is any movement of the air and radiation of heat if directly exposed to the sun. This net heat in or out must be balanced to maintain the body temperature in a relatively narrow range. The earth is subject to the same heat balance rules.
The heating of the earth due to the radiant heat of the sun is called the greenhouse effect - the earth and a greenhouse experience an increase in temperature due to trapped heat. However, the metaphor ends there. The greenhouse gets hot because the sun's photonic rays pass through the glass and heat the ground by radiation, the air next to the ground is warmed by conduction, and, as there is no convection to remove the heat, the "atmosphere" inside the greenhouse gets warm. The earth gets warm because the sun's photonic rays pass through the atmosphere and heat the earth's surface by radiation, just like the greenhouse only on a much larger scale. In order to stay in equilibrium, the earth must reradiate this heat back into space. The heat that is sent back out into space is absorbed in the atmosphere by several different gases - known as greenhouse gases. If it were not for this effect, the mean temperature of the earth would be about 33°C colder than it now is. The problem is that the higher the concentration of the greenhouse gases, the more heat is absorbed The more heat that is contained in the atmosphere, the hotter the earth must be to remain in equilibrium with the heat in the atmosphere. So it is essentially a heat transfer problem, albeit a complex one with multiple variables and chaotic turbulence.
Since the heat transfer of the greenhouse effect is from radiation, it is necessary to have a basic understanding of the wavelength and the energy of the sun's rays, which are made up of photons. A photon is "a quantum (a fixed elemental unit) of electromagnetic energy that has no mass and no charge," basically a packet of energy. It is one of the conundrums of physics that photons sometimes behave like particles and sometimes behave like waves. For the purpose of understanding greenhouse gases, it is better to use waves and to think of sound when considering wavelength and frequency. A high frequency sound has a short wave length; it doesn't go very far because the up and down movement of the wave in the air takes a lot of energy. A low frequency, long wavelength sound travels a long way. The sun sends out energy in waves that range from high energy, short wavelength waves called gamma rays to the low energy, long wavelength waves called microwaves. The higher energy, shorter wave length ultraviolet (above violet on the visible light spectrum) rays are mostly absorbed in the atmosphere; ozone (O3) is particularly good at absorbing UV rays. Visible rays (sunlight) and the lower energy, longer wavelength infrared (below red on the visible light spectrum) rays make it through the atmosphere and reach the earth. The sky looks blue because the particles in the air are of a size to interact with photons that have energy in the blue spectrum; this is called Rayleigh scattering. It is these penetrating infrared rays that are of concern.

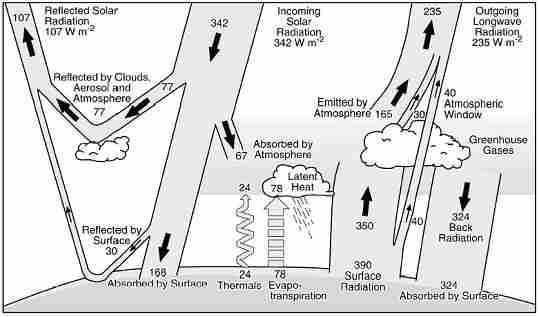
It is easier to visualize earth's radiation heat transfer with a diagram. The units used to measure the radiant flux energy are the same watts that are used for electricity; at the top of the atmosphere, the input energy is 342 watts per square meter (Wm-2), which is exactly balanced by a return of that radiant energy; 107 Wm-2 is reflected by the clouds or the surface and 235 Wm-2 is returned as a net result of the earth-atmosphere heat exchange interaction - and 107 + 235 = 342. The heated atmosphere sends 324 Wm-2 of infrared radiation back to the earth, called back radiation. The key to the global warming issue is that the earth must heat up so that it can return 390 Wm-2 to restore the balance. As the greenhouse gases build up, more infrared rays are absorbed in the atmosphere, and more are sent back to the earth as back radiation. Since greenhouse gases are causing the problem, the only solution afforded by physics is to reduce them.
Greenhouse Gases
According to the IPCC, greenhouse gases are "those gaseous constituents of the atmosphere, both natural and anthropogenic (man-made), that absorb and emit radiation at specific wavelengths within the spectrum of thermal infrared radiation emitted by the earth's surface, the atmosphere itself, and by clouds." The greenhouse gases, in essence, absorb the infrared heat because they have a linear dimension and geometric configuration that matches the wavelength of the infrared ray. The physics, which involves dipoles (the separation between positive and negative charges of the gas molecule) is much more involved, but it is sufficient for basic comprehension to consider the match between the size of the infrared wavelength and the dimensions of the gas - if they match, the gas absorbs the infrared heat and is a greenhouse gas. When the wave hits the gas, it transfers its photon energy to the gas, which vibrates; since molecular vibration is the basis for temperature, the gas heats up. The energized, hotter gas dissipates its heat through collisions with other gases; eventually the whole atmosphere heats up like a greenhouse.
The Kyoto protocol recognizes six greenhouse gases: carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), sulfur hexafluoride (SF6), hydrofluorocarbons (HFCs), and perfluorocarbons (PFCs). Water vapor is also a greenhouse gas since it absorbs infrared radiation; however, since the hydrologic (water science) cycle is fundamental to the operation of the climate system, it is not subject to direct anthropogenic influence and is not considered. The six greenhouse gases were chosen due to two factors, concentration and heat trapping capability. The concentration of the gas is directly proportional to the amount of infrared radiation (heat) it can absorb. The heat trapping capability refers to the relative efficiency of each molecule of the gas in absorbing radiation, this being a function of the geometry and charge configuration of the molecule. In order to afford a means of common measurement, all gases are normalized with a Global Warming Potential (GWP) relative to CO2 (which is assigned a GWP of 1.0) to bring them to the same standard for comparability of effects.
The last three "minor" greenhouse gases are frequently grouped together as the "F-gases" to indicate that they contain the element fluorine; taken together, they constitute less that 1% of the total greenhouse gas emissions. Sulfur hexafluoride is the product of heavy industry in the manufacture of semi-conductors. It is included because each SF6 molecule is the equivalent (GWP) of 23,900 molecules of CO2. HFCs and PFCs consist of a number of different compounds that were formulated to replace the CFCs (Chlorofluorocarbons, more commonly called CFCs) that were banned by the Montreal Protocol due to their ozone depleting effect. They are also largely included due to there high GWP values, which range between 140 and 11,700 for the HFCs and between 6,500 and 9,200 for the PFCs. In the 1950's, Barry Commoner, a prescient scientist considered by many the father of environmentalism, developed four laws of ecology. The irony of introducing greenhouse gases (HFC and PFC) to replace an ozone depleting substance (CFC) is direct evidence of his fourth law - "There is no free lunch" - every environmental solution (ozone depletion) has a cost (greenhouse gases).
Nitrous oxide (N2O) is the least known of the three "major" greenhouse gases, its provenance usually listed as "agricultural soil management." With a GWP of 310, it constitutes about 8% of the total greenhouse gas composition. The main culprit is fertilizer, which is about 10 percent nitrogen that must be added to the soil to compensate for the nitrogen removed with the harvest of the crop - about 100 pounds of nitrogen are removed with the harvest of every acre of corn. Fertilizer is necessary and sufficient to "manage" soil agricultural productivity. This added nitrogen is acted upon by the bacteria in the soil as a source of energy for their growth and reproduction - a process that is called nitrification, basically the conversion of ammonium (NH3) into nitrate (NO3). Nitrous oxide is a naturally occurring by-product of bacterial nitrification of the added nitrogen-based fertilizer. Not to get too technical but to be complete, there is also a process called denitrification in anaerobic (lacking oxygen) soils where bacteria reduce nitrate to gaseous nitrogen; denitrification, like nitrification, releases nitrous oxide as a by-product. Thus, as more crops are grown for the ever-expanding global population for either food, fodder or fuel (ethanol), more nitrogen enriched fertilizer must be used to reconstitute the depleted soil - and therefore more nitrous oxide results. The first law of ecology is - "Everything is connected to everything else" - the earth is such a complex and balanced ecosystem that every disturbance (added fertilizer) has far-reaching effects (greenhouse gases).
There are three primary sources of methane (CH4): enteric fermentation, natural gas systems and landfills. Taken together, they contribute more that three fourths of the total methane emissions in approximately equal shares. Enteric fermentation methane is from the normal digestion of food by ruminant animals, particularly cattle. Ruminants are named for the rumen, the first of their four stomachs - the repository for the fibrous material that they consume. Microbes in the rumen break down the tough cellulose as part of the digestive process; methane is a byproduct of that process that is expelled by the animal as exhalation. Over 95% of enteric fermentation methane is from beef and dairy cows. Other animals, including humans, produce the remainder of the enteric (intestinal) fermentation methane as flatulence. Methane is the primary constituent of natural gas that is widely used for heating and to generate electricity - some of this natural gas escapes into the atmosphere. Landfills are the largest of the three major sources of methane, comprising almost 40% of the total - the source is anaerobic bacterial decomposition of human trash. The second law of ecology applies to methane - "Everything must go somewhere" - there is no way to simply throw things (trash) away, because it will still be there and you have to live with the results (greenhouse gases).
And last but certainly not least is carbon dioxide, the scion of the industrial age and perhaps the harbinger of its demise; it makes up more than 80% of all greenhouse gasses - by definition it has a GWP of 1. The majority of carbon dioxide comes from the combustion of fossil fuel - oil, gas and coal. It is the energy released by the oxidation of hydrocarbons that is both the boon and the bane of the modern world. For example, the natural gas reaction is:
CH4 + 2O2 ------ > CO2 + H2O + energy
The level of CO2 in the atmosphere has historically been about 280 parts per million (ppm). It is now at 379 ppm, having risen by 80% since 1970. And this is, therefore, a problem that the world must face. The energy we use to make electricity and to operate vehicles is increasing greenhouse gas concentrations which are causing the earth to heat up. "Nature knows best" is the third law of ecology - every human made change is likely to be detrimental. Anthropogenic greenhouse gases are not likely beneficent.